Contractual basis
The contractual basis of InphA consists of, among other things:
- The 2nd amendment of the agreement regarding the collaboration in the area of medicinal products testing, dating 01 January 2021 and
- The memorandum of association dating 21 December 2012
InphA’s institutions
The following is a schematic representation of the company’s bodies:
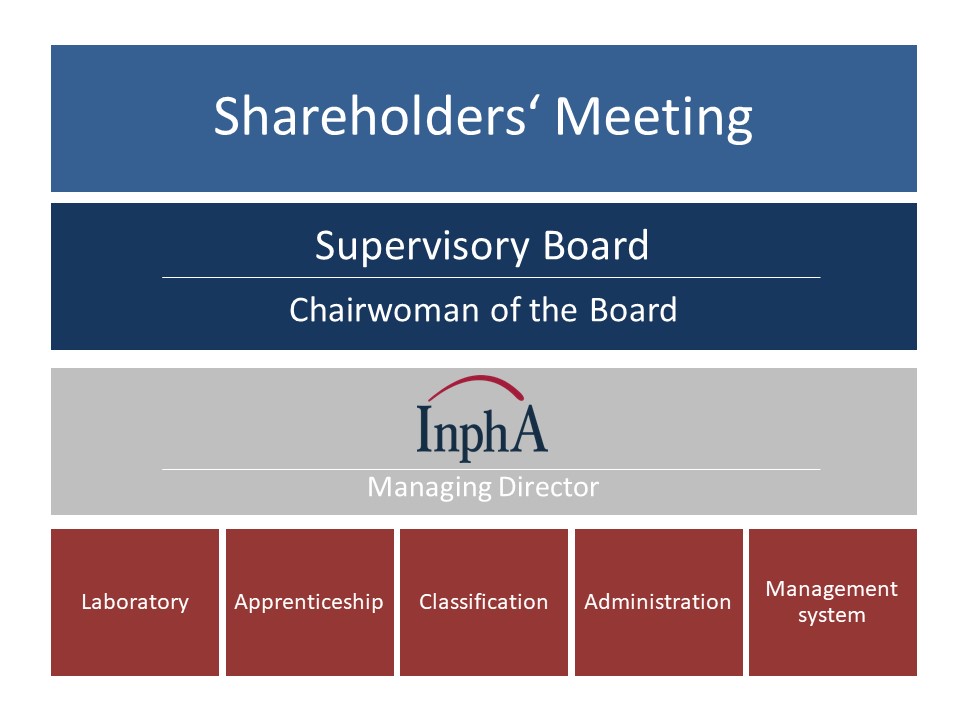
The shareholders’ meeting consists of representatives of the finance ministries of the shareholder States of Bremen, Hamburg, Hesse, Lower Saxony, Saarland and Schleswig-Holstein. All the states have equal rights, i.e. they hold an equal share of the nominal capital.
The supervisory board consists of the heads of the departments of the Federal Ministries of Health of the shareholding states. The office of the Chairman of the board rotates on a fixed schedule every two years. There are usually two meetings of the supervisory board and one shareholders’ meeting per year.
InphA is split into the following areas of responsibilities:
- Laboratory: This area consists of six scientific work groups, each with one laboratory manager (scientific employee) and one to three laboratory assistants.
- Apprenticeship: Usually, one to two trainees are employed as chemical laboratory assistants.
- Classification: This area is responsible for the classification of so called “borderline” products, which are usually products that are closely related to medicinal products like foodstuff, food supplements, medical devices etc.
- Administration: This area has a diverse range of tasks among others bookkeeping, purchasing, controlling, sample management (incoming goods, document check, requesting of analytical procedures and reference standards) and the creation of expense sheets and invoices.
- Management system: The task of the management representative according to ISO 17025 is assumed by a laboratory manager.
